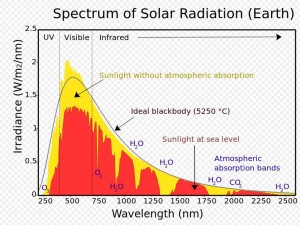
Sunlight is a powerful force. The sun emits radiation across most of the electromagnetic spectrum, including significant amounts of UV radiation. Although some of the sun’s radiation is absorbed by the Earth’s atmosphere, quite a bit of it reaches the earth’s surface (as you can see in the chart to the right). UV rays are capable of breaking molecular bonds in objects they impact. Most people are familiar with this effect on things like old paper products. As they are exposed to sunlight, the chemical bonds are broken down, causing the colors to fade. This color-changing effect is known as photodegradation.
However, the color of the object is not the only thing affected. The chemistry of the object is affected, so these effects are extremely important to the pharmaceutical, cosmeceutical, and nutriceutical industries. Chemical breakdowns in medications or vitamins need to be avoided, so it’s critical to make sure that pharmaceuticals and nutriceuticals remain stable for the duration of their expected shelf-life when exposed to light. This is done by testing the photostability of the product. Photostability can impact shelf life, handling, and packaging of the product. This testing is an important part of the drug development process. The photostability studies are usually performed in a sequential manner, with the drug/supplement substance being tested first. Afterwards, the product is tested in its immediate packaging and then its final marketing package that will be placed on retailers’ shelves. These tests can be repeated iteratively until they demonstrate that the drug is adequately protected from light.
Photostability testing standards for pharmaceuticals have been created by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (or ICH for short). The ICH standards include two options for lighting during testing. Option 1 involves using fluorescent D65, metal halide, or xenon lamps. These lamps do a very good job of mimicking outdoor daylight, but that comes with several downsides. First, these lamps produce a tremendous amount of heat. Any photostability testing chamber using these lights will need to be equipped with large fans to circulate enough air to keep the temperature stable. It also introduces the risk of the heat affecting the pharmaceuticals, making it difficult to tell if degradation in the drug is actually from the light, or rather from the heat the lamps produce. These lamps also have some other shortcomings. They have a short life span and need to be replaced regularly (every 750-1500 hours), and they have a fairly small area of illumination. Since the visual irradiance and UV outputs are tied together and can’t be independently controlled, these lamps overdose the samples with more UV radiation than recommended by the ICH specs.
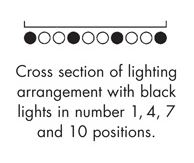
Option 2 in the ICH guidelines calls for two types of lighting: cool white fluorescent and near ultraviolet lamps. This is the option we implement in our photostability chambers. The graphic to the left shows how we arrange both the cool white fluorescent and black lights over each of the 4 shelves in the larger model (PST52SD). Using two different types of lights allows for independent control of the intensity of both illuminance (from the cool white lights) and UV irradiance (from the black lights). Since the UV lights can be controlled separately, the UV overdosing that may occur with option 1 lighting can be avoided. The light banks are wired to separate timers, so the UV lights can be turned off automatically when the desired UV testing dose is reached. Unlike in the option 1 lighting scheme, these lights produce very little heat, which mitigates the risk of the lights introducing thermal impacts in the study.
The ICH guidelines also include recommendations for testing temperature. Our photostability chambers can be run at temperatures from 20-45°C, which encompasses the entire range of long-term, intermediate, and accelerated testing specified by the ICH. Our chambers are built to your specifications, and come pre-validated to ICH Option 2 lighting standards, as well as the required temperature setting.
For more information, see our photostability chamber product page. To request a quote or get more information, visit our Contact Us page or call us at (800) 998-0500.